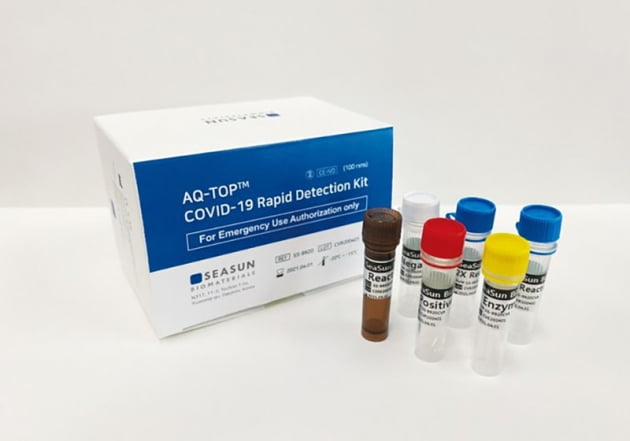
SeaSun Biomaterials, a diagnostic kit developer, has obtained emergency use approval (EUA) from the US Food and Drug Administration (FDA) for a new coronavirus infection (COVID 19) within 15 minutes.
On the 22nd, SeaSun Biomaterials said, "The company's 'AQ-TOP COVID-19 Rapid Detection Kit', which is a diagnostic kit for COVID-19, has received emergency approval from the US FDA on the 21st (local time)." This company has become a company that has been approved for two items as a COVID-19 diagnostic kit by the FDA. On the 27th of last month, SeaSun Biomaterials received emergency approval for the same polymerase chain reaction (RT-PCR) diagnostic kit from the FDA. The product that was approved for emergency use this time has reduced the time required for diagnosis from the previous 2 hours to within 15 minutes.
“This product can increase the number of viral genes in a short time by applying 'AQ-TOP isothermal amplification technology',” said an official from SeaSun Biomaterials. It will be used.”
관련전문:
https://www.hankyung.com/it/article/202005227063i
https://hellodd.com/?md=news&mt=view&pid=71900
http://www.econonews.co.kr/news/articleView.html?idxno=121574
https://www.fnnews.com/news/202005231124429815
https://newsis.com/view/?id=NISX20200522_0001034125&cID=13001&pID=13000

