SeaSun Biomaterials (CEO Hee-kyung Park) announced on the 29th that the U-TOP MSI Detection kit, which is a screening and diagnostic kit for colorectal and gastric cancer, was recognized for its original technology and recently obtained a patent.
This patent is a source technology that detects gene mutations through real-time PCR by detecting microsatellite instability (MSI) that exists as a repeat sequence in the human genome with a PNA probe. It encompasses exclusive rights to products. In particular, as the world's only real-time PCR-based MSI inspection product, the patent registration was decided in recognition of its technological advancement and novelty.
The PNA probe is a probe made of artificial DNA and can detect microsatellite with the highest accuracy in existence. The microsatellite is part of the genome (DNA) in which short nucleotide sequences consisting of 1 to 6 base pairs are repeated, and accounts for about 5% of the total DNA. When DNA is duplicated, errors frequently occur in this section, but if there is no protein to repair the mismatch error or if there is a problem with the function to correct the replication error, the number of repetitions of the short sequence may be less or more than normal, resulting in mutation.
Currently, the MSI test is mainly used for secondary diagnosis of nasal polyposis colorectal cancer, and is commonly found tumor-specific in gastric cancer, endometrial cancer, and ovarian cancer. As MSI has recently been confirmed in malignant melanoma, lung cancer, breast cancer, prostate cancer, and thyroid cancer, it is expected to become a basic means of implementing personalized treatment.
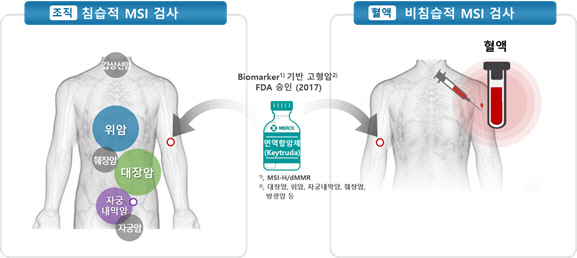
In particular, the U.S. Food and Drug Administration in May 2017 was MSI-H (high frequency microsatellite instability), which is impossible to resection or is used for secondary treatment of metastatic solid cancer. Zumab) was allowed to be prescribed, increasing interest in the MSI-H diagnostic criteria. This was the first case in the world that confirmed the prescription indication based on the genetic characteristics of the tumor, not the site where the tumor was expressed. Thanks to this atmosphere, the range of use of the MSI test is expected to expand to select treatments according to individual characteristics of each cancer type.
SeaSun Biomaterials said, “The MSI classification patent technology has already completed the PCT international application, and China and Vietnam, which are being examined, are about to register patents, and it is expected to be successfully registered in the US and Europe. After receiving the CE certification, it successfully debuted in the domestic market and recently received love calls from the US, Greece, and Italy, and started exporting.”
CEO Park Hee-kyung said, “The clinical trial is in progress to obtain a permission review that extends the MSI test to screening for endometrial and ovarian cancer.” “The product to expand tissue-based MSI test to blood-based will soon be developed, and biopsy will be It could be used to diagnose difficult or metastatic cancer early.” Blood tests are safe and simple, and can reduce the economic and time burden for cancer screening, and can contribute to early diagnosis and treatment of cancer.
관련전문:
http://www.edaily.co.kr/news/read?newsId=02269766622461040&mediaCodeNo=257&OutLnkChk=Y
http://www.viva100.com/main/view.php?key=20190430010010688
http://www.fnnews.com/news/201904261759537044
http://www.biospectator.com/view/news_view.php?varAtcId=7523
http://www.mdtoday.co.kr/mdtoday/index.html?no=353002

