
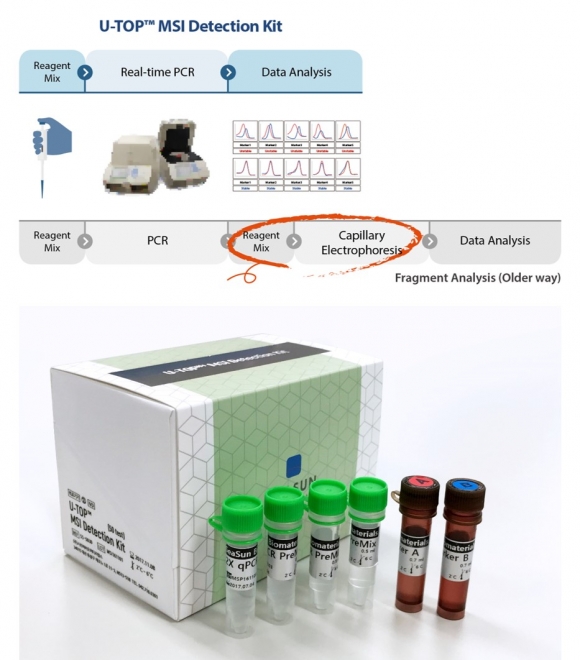
SeaSun Biomaterials has developed a diagnostic reagent that determines MSI instability through real-time PCR using a technology that detects changes in the length of a DNA sequence as a change in temperature. In particular, this product is a diagnostic product that can distinguish microsatellite stability (MSS), microsatellite instability-high (MSI-H), microsatellite instability-low (MSS-H), and microsatellite instability-low (MSS), which are detailed states of microsatellite. to be. Among the microsatellite markers, five markers of the Quasi guideline, which are highly sensitive, can be used to derive the same results as the existing MSI fragment analysis.
CEO Park Hee-kyung said, “The U-TOP MSI Detection Kit is the first product that uses a real-time polymerase chain reaction device, and is the first microsatellite instability diagnostic kit sold under the approval of the Ministry of Food and Drug Safety.” , "It is a product that demonstrates the technology commercialization capability of SeaSun Biomaterials in that it enables early market entry through the integrated approval review."
SeaSunbiomaterials is noting that in May, the FDA approved the immuno-cancer drug Kitruda as the first cancer treatment that can be prescribed to patients with specific genetic indicators (biomarkers) regardless of the site of cancer. This is because the U-TOP MSI Detection Kit is the first in vitro diagnostic device that can diagnose MSI, the biomarker that determines the prescription of Kitruda. Specifically, it is possible to prescribe Kitruda for adults and children with high microsatellite instability (MSI-H) or mismatch repair deficient (dMMR) characteristics, unresectable or metastatic solid tumors. Tumors with high microsatellite instability (MSI-H) are commonly found in colon cancer, endometrial cancer, and gastrointestinal cancer, but can also appear in breast cancer, prostate cancer, bladder cancer, and thyroid cancer.
A company official said, "In the case of organ-centered classification of indications based on the location of existing tumors, the location of the first cancer is an important classification criterion, so if imaging tests such as tomography are essential, classification of indications based on biomarkers. In the case of biomarkers, the importance of in vitro diagnostic tests according to the type of biomarker has increased. “In the future, we plan to enter the existing cancer classification diagnosis (colorectal cancer, gastric cancer, endometrial cancer, etc.) market by linking the biomarker market of immunotherapy drugs such as Kitruda "I emphasized.
SeaSun Biomaterials plans to continue developing products that diagnose biomarkers that can improve the treatment efficiency of cancer patients, not just the MSI diagnostic kit. It is conducting clinical trials by completing the development of a diagnostic kit for EBV (Epstein-Barr virus) related to gastric cancer, a diagnosis of Helicobacter pylori and an antibiotic resistance gene diagnosis product.
관련원문:
http://www.biospectator.com/view/news_view.php?varAtcId=4070
http://www.edaily.co.kr/news/NewsRead.edy?SCD=JG61&newsid=02745366616064712&DCD=A00706&OutLnkChk=Y